Do Polyurea Coatings Hold Up to Bigfoot Blood?

SPRAY FOAM MAGAZINE – So, what is with all the recent Bigfoot sightings? With reports of aliens among us, this becomes a serious topic. All joking aside here, this is an important issue, especially for those who are transitioning from spray foam work to polyurea spray applications.
I can’t begin to tell you how many contacts I get a week from people wanting to know if a polyurea system will hold up to a certain chemical, or a strange, or common material. This is a good thing, as they are concerned about the application work, but more specifically and often, they inquire as to whether polyurea will hold up to just a certain pH. Seems like a simple question, right? While I really do not mind receiving the contacts and inquiries, it is not really a question that is so easy to answer because not enough information has been provided.
Acidic & Alkaline Solutions
First, let’s look at the pH part of the question. In our chemistry world, pH is the “Potential Hydrogen”, measured as the –log10 (H+) hydrogen ion concentration. Or in the case of basic/alkaline conditions, we use the hydroxide (OH-) ion concentration and subtract from 14. Sounds complicated right, but hold on a minute, it’s pretty “simple” in a way. You can also check Wikipedia for more info to brush up on your chemistry.
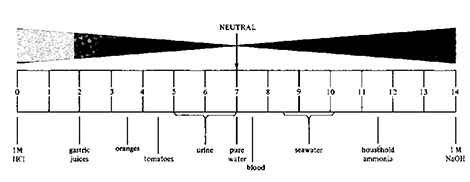
For pH, we use a scale as shown below. This scale runs from 0 – 14, with 7 being neutral. Anything less than 7 is acidic, and that above 7 is basic or alkaline. The lower the number, the more acidic, while the higher the number, the more alkaline. The change in pH numbers from one to the next represents about a 10-fold change in percent “concentration.”
For purposes of this discussion, from an acid standpoint, simply we have either inorganic (mineral) or organic-based acids. Examples of inorganic acids would include hydrochloric (HCl), sometimes called muriatic acid, nitric (HNO3) also referred to as aqua fortis, and then there is a fuming version of this by the way; it’s not mad, but if you saw it you would think it was. There is also phosphoric (H3PO4) and sulfuric (H2SO4) acid to name a few.
But not to complicate matters, if we look at acids based upon the halogen series, and that being HCl, HBr (hydrobromic) and, HF (hydrofluoric), these are most definitely not the same. A similar pH of each one of these will have significantly different results in exposure.
Then, there are organic acids such as common ones that include acetic, lactic, and citric that may be encountered in polyurea coating and lining work. Organic acids may tend to be a little more aggressive than mineral-based at the same pH level.

A dilute trichloroacetic acid solution, pH 2 let’s say, will eat right through most polyurea systems, while a dilute acetic acid, same pH as above, will be fine in exposure. For example, let’s say that we have a hydrochloric acid solution at a pH of 2, and that represents about a 0.036% aqueous HCl solution. A pH of 1 is about 0.36%, while ~3.6% solution is a pH of about 0. If you note, most polyurea systems will hold up to room temperature exposure of a 10% HCl solution, a pH value of < 0.
Acids and bases can also be used in other aqueous solutions to aid in the dissolution of certain chemical compounds or salts. While this “acid” or “base” may not be disclosed specifically on the Safety Data Sheet (SDS), look at the pH noted of the material, and this can provide some additional information. So chemicals used as well as pH, is an important combination to know.
I will note here that polyurea systems in general hold up better to alkaline conditions and exposure as compared to an acidic environment, but this does not mean they cannot be used in acidic environments; we just need to know a little more information. A specific topcoat may be required.
Oxidizing Chemicals
Oxidizing materials such as solutions of Chlorine, NaClO (Sodium Hypochlorite - bleach), NaBrO (Sodium Hypobromite) and H2O2 (Hydrogen Peroxide, and other peroxides) DO NOT play well with aromatic-based polyurea systems. Ah yes, sunlight is a strong oxidizing agent.
Many of these types of oxidizing chemicals are used as cleaning agents in the food processing industry, along with an acidic solution. In most cases when these materials are used in their proper dilution factor, all is well. However, when used in full concentration, or not proper dilution, subsequent failures can and will occur. Remember, most of the food processing equipment is made of high-grade stainless steel, which is very corrosion-resistant.
In general, aliphatic-based polyurea systems hold up much better to oxidizing agents than do the aromatic-based polyurea systems. Exposure to these oxidizing chemicals is one major reason to proceed with caution when considering lining swimming pools with an aromatic polyurea system. As a side note here, aliphatic “modified” polyurea systems, which are good materials, are still considered aromatic-based when it comes to most chemical and UV exposure.
Other chemical Exposure
Other chemicals such as hydrocarbons, alcohols, fuel, oils, solvents, certain amines, etc. can all have different effects depending upon the molecular weight of the specific chemical, the backbone on the molecule, and the type of polyurea system required. In most cases with these types of chemical exposure, softening and swelling (or cracking) of the exposed polyurea system can be noted. This is due to the permeability of the polyurea system being used.
For example, a polyurea bedliner system is great for truck bedliner applications, which it was designed for. However, that system may in fact NOT work for a tank lining or constant chemical exposure applications. Yes, it is a polyurea system, but formulation wise it is not designed for that type of application in constant immersion.
Temperature and time of Exposure
The next consideration will be exposure temperature. As one is aware, when things heat up, they start to move faster and work more efficiently. The same is true for chemical exposure. Certain polyurea systems may hold up well to a chemical exposure at 77°F (25°C) but will fail if that temperature is elevated, say even to 120°F (~50°C). Knowing this as well, is a valuable piece of information that is required.
For certain chemicals, a polyurea system may be fine for “splash and spill” as noted in secondary containment work. That same chemical in constant immersion will be detrimental to the polyurea systems. There should be a written prescribed procedure for cleanup, and followed of course, in case of a splash and spill situation.

Also, polyurea is a technology, as there are numerous formulation possibilities, and not all are the same. One needs to know what polyurea system is required, and if aromatic or aliphatic-based. Don’t just use “polyurea,” as I see so often in requests on certain social media sites. It makes my head hurt at times.
To answer the question, you need to ask more questions and gather important information. This will ensure a successful polyurea project, and that is what we want. This will also let you know if you need to “walk away,” and don’t be afraid to do that either. With so many chemicals in this world, it is impossible to evaluate all of them. Your supplier should have a general chemical resistance guide for their products.
One good example of important information can be found on the chemicals in use in the SDS, Section 3: Composition/Information on Ingredients. This can be provided to the polyurea system supplier to confirm if their system will hold up to that chemical, and exposure temperature. Work with your supplier, not against them. Most importantly, get the information related to chemicals and exposure temperature in writing and document that in the work proposal. This will protect all parties involved in the end.
So now as far as Bigfoot blood, I would have to assume that it is similar in make-up to other mammal “blood,” and yes, polyurea should hold up well to that exposure. For alien blood, it is probably highly acidic, well from what I have seen in the movies anyway. So, one should use caution here. But if in doubt, you bring me a Bigfoot, or an alien and we can test if need be.
For use by SprayFoamMagazine.com & Spray Foam Magazine













